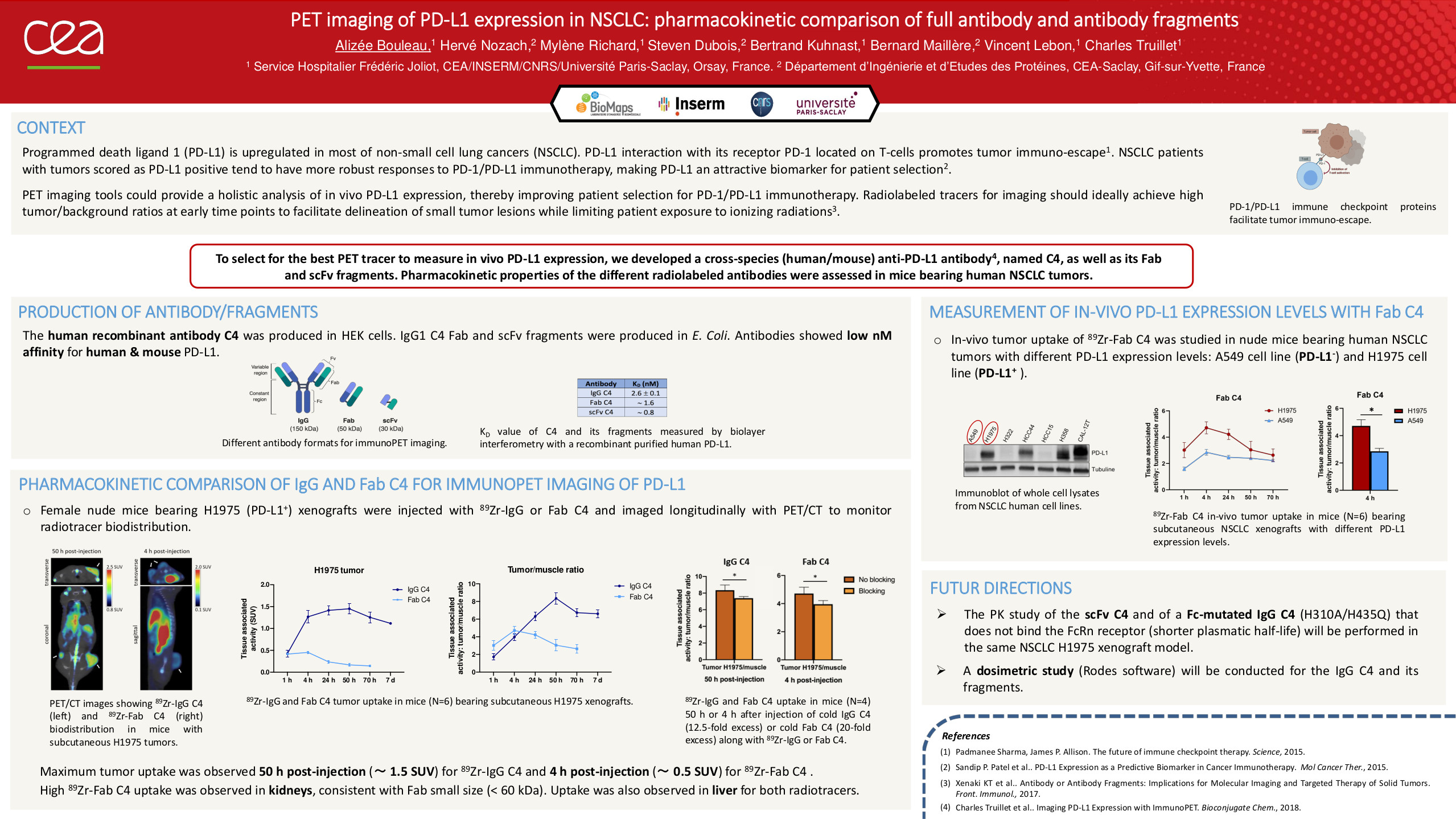
The interaction of programmed death ligand 1 (PD-L1) with its receptor PD-1 located on T-cells promotes tumor cell immuno-escape. Non-small cell lung cancer (NSCLC) patients with tumors scored as PD-L1 positive tend to have more robust responses to PD-1/PD-L1 immunotherapy, making PD-L1 an attractive biomarker for patient selection. Immunohistochemistry (IHC) on tumor biopsies is currently the reference standard for assessing PD-L1 expression. However, this process faces several issues, including intra-tumoral PD-L1 heterogeneity, or primary vs. metastatic biopsies. PET imaging tools may provide a more holistic analysis of in vivo PD-L1 expression, thereby improving patient selection for PD-1/PD-L1 immunotherapy. PET tracers should ideally achieve high tumor-to-background ratios at early time points, while being rapidly cleared. For this purpose, we developed a cross-species (human/mouse) anti-PD-L1 antibody (named C4), as well as its Fab and scFv fragments. The best PD-L1 PET tracer will be selected by comparing pharmacokinetics, biodistribution, and dosimetry of the antibody/antibody fragments radiolabeled with 89Zr or 18F in nude mice bearing subcutaneous human NSCLC tumors. Murine 3D imaging-based dosimetry analysis will be performed using the RODES software developed by the IRSN Institute.